How Many Electrons Does Bromine Have
Next Genwave
Mar 09, 2025 · 6 min read
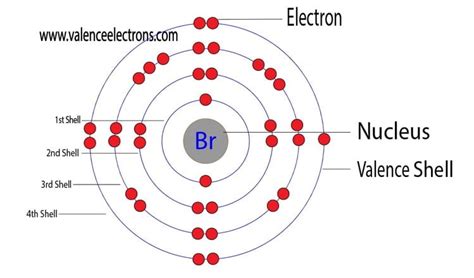
Table of Contents
How Many Electrons Does Bromine Have? A Deep Dive into Atomic Structure
Bromine, a fascinating element with a rich history and diverse applications, holds a unique place in the periodic table. Understanding its atomic structure, particularly the number of electrons it possesses, is fundamental to grasping its chemical behavior and properties. This article delves deep into the world of bromine, exploring not just the simple answer to the question "how many electrons does bromine have?", but also the underlying principles of atomic structure and how these principles determine bromine's characteristics.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we pinpoint the number of electrons in bromine, let's revisit the basics of atomic structure. Every atom is composed of three fundamental subatomic particles:
-
Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the element's atomic number and dictates its identity.
-
Neutrons: Neutrally charged particles residing in the atom's nucleus alongside protons. The number of neutrons can vary within the same element, leading to isotopes.
-
Electrons: Negatively charged particles orbiting the nucleus in specific energy levels or shells. These electrons are responsible for chemical bonding and determine the element's chemical reactivity.
The number of protons and electrons in a neutral atom are always equal, ensuring a balanced charge. This neutrality is crucial for the stability of the atom.
Determining the Number of Electrons in Bromine
Bromine's atomic number is 35. This means a neutral bromine atom contains 35 protons in its nucleus. Since the number of protons and electrons in a neutral atom are equal, a neutral bromine atom also possesses 35 electrons.
This simple fact forms the bedrock of understanding bromine's chemical behavior. The arrangement of these 35 electrons in various energy levels determines its reactivity and the types of bonds it can form with other atoms.
Electron Configuration: Unveiling Bromine's Electronic Structure
The electrons within a bromine atom are not randomly scattered. They occupy specific energy levels or shells, organized according to the principles of quantum mechanics. This arrangement, known as the electron configuration, is crucial for understanding the atom's properties.
The electron configuration of bromine is 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁵. Let's break this down:
- 1s²: Two electrons occupy the first energy level (n=1), specifically the s orbital.
- 2s²2p⁶: Eight electrons occupy the second energy level (n=2), with two in the s orbital and six in the three p orbitals.
- 3s²3p⁶: Eight electrons occupy the third energy level (n=3), similar to the second level.
- 4s²3d¹⁰: Eighteen electrons occupy the fourth energy level (n=4). This includes two electrons in the s orbital and ten electrons filling the five d orbitals. Note the filling order: 4s fills before 3d.
- 4p⁵: Five electrons occupy the p orbitals of the fourth energy level.
This specific arrangement of electrons explains bromine's chemical properties. The incomplete 4p subshell (with only five electrons instead of a full six) is responsible for bromine's high reactivity. It readily accepts one more electron to achieve a stable octet configuration (eight electrons in its outermost shell), making it a strong oxidizing agent.
Bromine's Chemical Properties: A Consequence of its Electron Configuration
The presence of 35 electrons, particularly their arrangement in the electron configuration, directly influences bromine's chemical behavior:
-
Reactivity: The incomplete outermost shell (4p⁵) makes bromine highly reactive. It readily forms chemical bonds to achieve a stable octet, either by gaining an electron or sharing electrons with other atoms.
-
Oxidizing Agent: Bromine's tendency to gain an electron makes it a strong oxidizing agent. It readily oxidizes other substances, accepting electrons from them and becoming reduced in the process.
-
Formation of Compounds: Bromine forms a wide variety of compounds with other elements, including ionic compounds (with metals) and covalent compounds (with nonmetals). The strength and nature of these bonds are dictated by the interactions between bromine's electrons and those of other atoms.
-
Halogen Properties: Bromine is a halogen, a group of elements characterized by their high reactivity and tendency to form -1 ions. Its properties are consistent with other halogens like chlorine and iodine, although the reactivity varies across the group.
-
Physical States: The number of electrons and the resulting intermolecular forces between bromine atoms influence its physical state. At room temperature, bromine exists as a reddish-brown liquid, a characteristic influenced by the electron configuration and interatomic interactions.
Isotopes of Bromine and Electron Count
While the number of electrons in a neutral bromine atom is always 35, the number of neutrons can vary, leading to different isotopes of bromine. Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons. The most common isotopes of bromine are bromine-79 and bromine-81. Both isotopes have 35 electrons, but they differ in their mass number (protons + neutrons). The difference in neutron count affects the atom's mass but not its chemical behavior. The chemical properties are primarily determined by the electron configuration.
The existence of isotopes further emphasizes the importance of understanding the atomic number (number of protons, which equals the number of electrons in a neutral atom) in determining an element's identity and chemical properties.
Bromine's Importance and Applications
Bromine's unique properties, stemming directly from its 35 electrons and resulting electron configuration, have led to its widespread use in various applications:
-
Flame Retardants: Bromine compounds are added to materials to reduce their flammability, improving fire safety.
-
Agricultural Chemicals: Certain bromine compounds are used as pesticides and fumigants in agriculture.
-
Medical Applications: Bromine compounds have found use in some medications and medical treatments.
-
Water Treatment: Bromine is used in some water purification processes to disinfect and remove impurities.
-
Dyes and Colorants: Bromine compounds are used in the production of various dyes and colorants.
-
Photography: Historically, bromine compounds have been used in photographic processes.
Conclusion: The Significance of 35 Electrons
The seemingly simple answer – bromine has 35 electrons – opens a door to a deeper understanding of atomic structure, chemical bonding, and the rich diversity of chemical properties. The number of electrons, their arrangement in specific energy levels, and their interactions with other atoms are all fundamental to understanding why bromine behaves the way it does. Its reactivity, its ability to form diverse compounds, and its widespread applications are all a direct consequence of its 35 electrons and their unique configuration. This detailed exploration highlights the fundamental importance of understanding atomic structure in comprehending the behavior and utility of elements, a crucial aspect of chemistry and related fields. From the fundamental subatomic particles to the vast array of applications, the 35 electrons in bromine tell a compelling story of the power of atomic structure.
Latest Posts
Latest Posts
-
31 3 As A Mixed Number
Mar 09, 2025
-
3 N 4 1 2 6n 4
Mar 09, 2025
-
What Is 1 3 Divided By 2
Mar 09, 2025
-
0 996 Rounded To The Nearest Hundredth
Mar 09, 2025
-
1 2 Times 1 2 Times 1 2
Mar 09, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Does Bromine Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
