How Many Protons Does Gallium Have
Next Genwave
Mar 07, 2025 · 6 min read
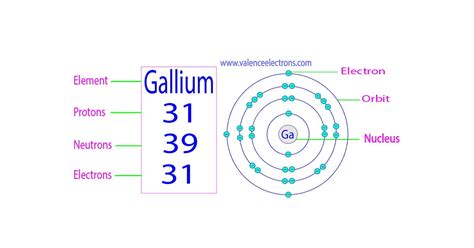
Table of Contents
How Many Protons Does Gallium Have? A Deep Dive into Atomic Structure
Gallium, a fascinating element with a curious melting point just above room temperature, holds a unique place in the periodic table. But the fundamental question that unlocks understanding its properties lies in its atomic structure: How many protons does gallium have? The answer, as we'll explore in detail, is 31. This seemingly simple number holds the key to understanding its chemical behavior, its place in the periodic table, and its various applications. This article will not only answer this question but also delve deep into the broader concepts of atomic structure, isotopes, and the significance of proton number in defining an element.
Understanding Atomic Structure: The Nucleus and Beyond
To understand why gallium has 31 protons, we need to grasp the basics of atomic structure. An atom, the fundamental building block of matter, consists of three primary subatomic particles:
-
Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the element. This is what distinguishes gallium from all other elements.
-
Neutrons: Neutral particles (no charge) also found in the nucleus. The number of neutrons can vary within an element, leading to isotopes.
-
Electrons: Negatively charged particles orbiting the nucleus in electron shells. The number of electrons usually equals the number of protons in a neutral atom.
The nucleus, containing protons and neutrons, forms the dense core of the atom, accounting for almost all of its mass. The electrons, much lighter than protons and neutrons, occupy the space surrounding the nucleus. The arrangement of these electrons determines the atom's chemical properties and how it interacts with other atoms.
The Significance of the Proton Number (Atomic Number)
The atomic number of an element is the number of protons in the nucleus of an atom of that element. This number is unique to each element and is crucial for its identification. It's the fundamental property that distinguishes gallium (atomic number 31) from all other elements. Elements are arranged in the periodic table in order of increasing atomic number, reflecting the systematic increase in the number of protons.
This number directly dictates the element's chemical behavior. The number of protons determines the positive charge of the nucleus, which in turn dictates the number of electrons needed to achieve a neutral atom. This electron configuration dictates how the atom will interact with other atoms through chemical bonding. For gallium, its 31 protons mean it has 31 electrons in a neutral atom, leading to its specific electronic configuration and thus its chemical properties.
Gallium: A Closer Look at Element 31
Gallium (Ga), a silvery-white metal, is a post-transition metal located in Group 13 (Boron group) and Period 4 of the periodic table. Its unique characteristic, as mentioned earlier, is its exceptionally low melting point, making it liquid at temperatures slightly above room temperature.
Key Properties of Gallium:
- Atomic Number: 31
- Atomic Mass: Approximately 69.723 amu (atomic mass units)
- Melting Point: 29.76 °C (85.57 °F)
- Boiling Point: 2204 °C (3999 °F)
- Density: 5.91 g/cm³
- Appearance: Silvery-white, lustrous solid
The low melting point of gallium is due to its relatively weak metallic bonding. This weak bonding makes gallium relatively easy to melt and explains its unique properties.
Isotopes of Gallium: Variations in Neutron Count
While the number of protons defines gallium, the number of neutrons can vary, leading to different isotopes. Isotopes are atoms of the same element that have the same number of protons but a different number of neutrons. This means they have the same atomic number but different atomic masses.
Gallium has two naturally occurring stable isotopes:
-
⁶⁹Ga: This isotope comprises about 60.1% of naturally occurring gallium and has 38 neutrons (31 protons + 38 neutrons = 69 nucleons).
-
⁷¹Ga: This isotope accounts for the remaining 39.9% and has 40 neutrons (31 protons + 40 neutrons = 71 nucleons).
Although these isotopes have different numbers of neutrons, their chemical properties remain essentially the same because the number of protons (and therefore electrons) is identical. The difference lies primarily in their mass and, in some cases, their radioactive properties. Some synthetic, radioactive isotopes of gallium also exist, but these are not found naturally.
The Importance of Protons in Chemistry and Physics
The number of protons is paramount not only for identifying an element but also for understanding its chemical behavior and its role in various scientific fields.
Chemical Reactions and Bonding:
The arrangement of electrons in the outermost shell (valence electrons) governs how an atom interacts with other atoms. This arrangement is a direct consequence of the number of protons. Gallium's 31 protons dictate its three valence electrons, influencing its bonding behavior and resulting in compounds with oxidation states of +1 and +3. This explains why gallium forms a variety of compounds and alloys.
Nuclear Physics and Radioactivity:
In nuclear physics, the number of protons and neutrons plays a crucial role in determining an atom's stability and radioactive properties. The ratio of protons to neutrons influences the strong nuclear force holding the nucleus together. Isotopes with unstable proton-neutron ratios are radioactive, undergoing decay processes to achieve a more stable configuration. Some radioactive isotopes of gallium, though not naturally occurring, find applications in medical imaging and nuclear medicine.
Applications of Gallium: Leveraging its Unique Properties
The properties of gallium, determined by its 31 protons and consequent electron configuration, have led to numerous applications in various fields:
-
Semiconductors: Gallium arsenide (GaAs) is a crucial semiconductor material used in high-speed integrated circuits, LEDs (light-emitting diodes), solar cells, and other electronic devices. Its properties make it ideal for these applications, surpassing silicon in certain areas.
-
Alloys: Gallium alloys are used in low-melting-point solders, dental amalgams, and other applications requiring materials with specific melting points.
-
Medicine: Some gallium isotopes are used in medical imaging and radiotherapy. Gallium-67 is used as a radiotracer to detect infections and tumors.
-
Other applications: Gallium is also used in mirrors, producing a highly reflective surface. Its unique properties make it a valuable component in a wide array of technologies.
Conclusion: The Proton's Power in Defining Gallium
In conclusion, gallium's defining characteristic, its 31 protons, dictates virtually all its properties and applications. This simple number is the key to understanding its place in the periodic table, its chemical behavior, its isotopic variations, and its wide range of technological applications. From its unusual melting point to its role in semiconductor technology and medical imaging, gallium's story is a testament to the power of a single atomic number. The seemingly simple answer – 31 protons – opens the door to a complex and fascinating world of atomic structure and its profound implications. Further exploration into the behavior of elements, particularly focusing on their atomic structure, will continue to unveil new advancements in various scientific and technological fields.
Latest Posts
Latest Posts
-
X 3 X 2 16x 16
Mar 09, 2025
-
How To Find Class Midpoints In Statistics
Mar 09, 2025
-
2 1 4 As An Improper Fraction
Mar 09, 2025
-
What Is 1 5th Of 15
Mar 09, 2025
-
What Is 3 375 As A Fraction
Mar 09, 2025
Related Post
Thank you for visiting our website which covers about How Many Protons Does Gallium Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
