How Many Neutrons Does Silver Have
Next Genwave
Mar 10, 2025 · 5 min read
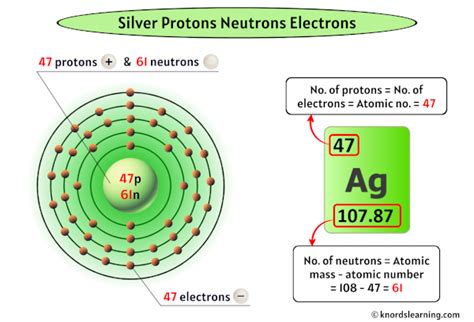
Table of Contents
How Many Neutrons Does Silver Have? Understanding Isotopes and Atomic Structure
Silver, a lustrous white metal prized for its beauty and conductivity, presents a fascinating case study in atomic structure. The seemingly simple question, "How many neutrons does silver have?" reveals a deeper complexity involving isotopes and the nuances of nuclear physics. This comprehensive guide will explore the answer, delving into the concepts of atomic number, mass number, isotopes, and their implications for understanding silver's properties.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we dive into silver's neutron count, let's establish a foundational understanding of atomic structure. Every atom consists of three subatomic particles:
-
Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the element; all silver atoms have 47 protons. This is known as the atomic number.
-
Neutrons: Neutral particles (no charge) also residing in the nucleus. Along with protons, they contribute to the atom's mass.
-
Electrons: Negatively charged particles orbiting the nucleus in shells or energy levels. The number of electrons typically equals the number of protons in a neutral atom.
Isotopes: The Key to Variable Neutron Numbers
The answer to "how many neutrons does silver have?" isn't a single number. This is because silver, like many elements, exists as a mixture of isotopes. Isotopes are atoms of the same element (same number of protons) but with different numbers of neutrons. This difference in neutron number affects the atom's mass but not its chemical properties significantly.
The mass number of an atom is the sum of its protons and neutrons. Since the number of protons is constant for a given element (47 for silver), variations in mass number directly reflect variations in the neutron count.
Silver's Isotopes: A Closer Look
Silver has two naturally occurring stable isotopes:
-
Silver-107 (¹⁰⁷Ag): This isotope accounts for approximately 51.8% of naturally occurring silver. It has 47 protons and 60 neutrons (107 - 47 = 60).
-
Silver-109 (¹⁰⁹Ag): This isotope makes up roughly 48.2% of naturally occurring silver. It contains 47 protons and 62 neutrons (109 - 47 = 62).
Therefore, a straightforward answer to "How many neutrons does silver have?" is it depends on the isotope. It can have either 60 neutrons (¹⁰⁷Ag) or 62 neutrons (¹⁰⁹Ag).
Calculating Average Neutron Number
Since naturally occurring silver is a mixture of these two isotopes, we can calculate a weighted average neutron number. This represents the average number of neutrons per silver atom in a naturally occurring sample:
(0.518 * 60 neutrons) + (0.482 * 62 neutrons) = 61.02 neutrons
This calculation gives us an average neutron number of approximately 61.02. However, it's crucial to remember this is an average; no single silver atom possesses 61.02 neutrons.
Other Silver Isotopes: Radioactive and Unstable
While ¹⁰⁷Ag and ¹⁰⁹Ag are stable, several other silver isotopes exist, but they are radioactive and unstable. These isotopes decay over time, transforming into other elements through processes like beta decay or alpha decay. These radioactive isotopes have different neutron counts than the stable ones and are not naturally occurring in significant quantities.
Impact of Neutron Number on Silver's Properties
The number of neutrons in a silver atom subtly influences its properties. While the chemical behavior is primarily determined by the number of protons and electrons (which define its electron configuration and thus its reactivity), the neutron number affects:
-
Mass: The differing neutron counts in ¹⁰⁷Ag and ¹⁰⁹Ag contribute to the overall atomic mass of silver, influencing its density and other physical properties.
-
Nuclear Stability: The neutron-to-proton ratio is crucial for nuclear stability. The stable isotopes of silver maintain a balance that prevents radioactive decay. Unstable isotopes have neutron-to-proton ratios that make them prone to radioactive decay.
-
Nuclear Reactions: The neutron number directly influences how a silver atom interacts in nuclear reactions, such as neutron capture or fission.
Applications of Silver and Isotopic Considerations
Silver's unique properties, stemming from its atomic structure and isotopic composition, make it valuable in various applications:
-
Jewelry and Ornaments: Silver's beauty and malleability have led to its widespread use in jewelry and decorative items for centuries. The isotopic composition has a negligible effect on these applications.
-
Electronics: Silver's excellent electrical conductivity makes it essential in electronic components, including circuits and contacts. The slight mass difference between the isotopes has minimal impact on this application.
-
Photography: Silver halides were historically crucial in photographic film and printing processes. Isotopic composition is largely irrelevant in this context.
-
Medicine: Silver's antimicrobial properties make it useful in wound dressings and medical devices. Again, the isotopic composition doesn't significantly affect the efficacy.
-
Catalysis: Silver is a catalyst in certain chemical reactions, and the isotopic composition can have subtle effects on catalytic activity, but these effects are often negligible in practical applications.
Conclusion: A Deeper Understanding of Silver's Atomic Composition
The question of how many neutrons silver has highlights the complex relationship between isotopes and atomic properties. While silver commonly possesses 60 or 62 neutrons depending on the isotope (¹⁰⁷Ag or ¹⁰⁹Ag respectively), considering the weighted average neutron number offers a broader understanding of its naturally occurring form. The number of neutrons significantly influences the mass and nuclear stability of silver, although it plays a relatively minor role in its chemical behavior and common applications.
This detailed exploration emphasizes the importance of understanding atomic structure, isotopes, and their implications for various scientific and technological fields. Further research into the radioactive isotopes of silver and their decay mechanisms can reveal even more intricate aspects of silver's nuclear behavior and potential applications in areas like nuclear medicine and scientific research. The simplicity of the initial question belies the depth of scientific understanding required for a complete answer.
Latest Posts
Latest Posts
-
What Is 75 Celsius In Fahrenheit
Mar 10, 2025
-
What Is 1 4 Of 4
Mar 10, 2025
-
5 4 As A Mixed Number
Mar 10, 2025
-
What Is 3 2 Divided By 2
Mar 10, 2025
-
What Is 12 25 As A Percent
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about How Many Neutrons Does Silver Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
