How Many Protons Does Neon Have
Next Genwave
Mar 07, 2025 · 5 min read
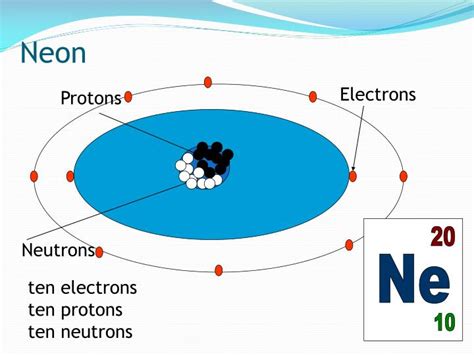
Table of Contents
How Many Protons Does Neon Have? A Deep Dive into Atomic Structure
Neon, the vibrant gas that illuminates our signs and plays a crucial role in various technologies, holds a fascinating place in the periodic table. Understanding its atomic structure, particularly the number of protons it contains, is key to comprehending its properties and behavior. This comprehensive guide will delve into the world of neon, exploring not just its proton count, but also its place within the broader context of atomic theory and its practical applications.
Understanding Atomic Structure: The Foundation of Neon
Before we pinpoint the number of protons in neon, let's establish a fundamental understanding of atomic structure. An atom, the basic building block of matter, is composed of three primary subatomic particles:
- Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element; it's the element's atomic number.
- Neutrons: Neutrally charged particles also found in the nucleus. The number of neutrons can vary within an element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. The number of electrons typically equals the number of protons in a neutral atom.
The nucleus, containing protons and neutrons, constitutes the vast majority of an atom's mass, while electrons occupy the relatively vast space surrounding the nucleus. This arrangement dictates the atom's chemical properties and how it interacts with other atoms.
Neon's Atomic Number: The Key to its Proton Count
Neon's atomic number is 10. This fundamental number, found on the periodic table, directly indicates the number of protons present in a neon atom's nucleus. Therefore, a neon atom has 10 protons. This is a defining characteristic of neon, distinguishing it from all other elements. No other element possesses exactly 10 protons.
Isotopes: Variations in Neutron Count
While the number of protons remains constant for a given element, the number of neutrons can vary. Atoms of the same element with differing neutron counts are called isotopes. Neon has three naturally occurring isotopes:
- Neon-20 (²⁰Ne): This is the most abundant isotope, comprising about 90.48% of naturally occurring neon. It has 10 protons and 10 neutrons.
- Neon-21 (²¹Ne): A less common isotope, making up around 0.27% of natural neon. It has 10 protons and 11 neutrons.
- Neon-22 (²²Ne): Another naturally occurring isotope, present in approximately 9.25% of natural neon. It has 10 protons and 12 neutrons.
These isotopes exhibit similar chemical behavior due to their identical number of protons and electrons, but their slightly different masses can lead to subtle differences in physical properties.
The Significance of Neon's 10 Protons
The presence of 10 protons in neon's nucleus is not merely a numerical fact; it has profound implications:
-
Chemical Inertness: Neon's electronic configuration, with a full outer electron shell (2s²2p⁶), makes it exceptionally stable and unreactive. This full octet renders it chemically inert, meaning it rarely forms chemical bonds with other elements. This inertness is directly linked to its 10 protons and resulting electron arrangement.
-
Noble Gas Properties: Neon belongs to Group 18 of the periodic table, known as the noble gases. These elements are all characterized by their chemical inertness, largely due to their complete outer electron shells. Neon's 10 protons play a critical role in achieving this stable electronic configuration.
-
Physical Properties: Neon's 10 protons contribute to its characteristic physical properties, including its low density, colorless nature, and its ability to emit a characteristic reddish-orange glow when electrically excited. These properties are intricately connected to its atomic structure and the interactions between its electrons and protons.
Applications of Neon: From Lighting to Lasers
Neon's unique properties, stemming directly from its atomic structure and the presence of its 10 protons, have led to a range of practical applications:
-
Neon Lighting: Neon's ability to emit light when subjected to an electric current is the basis of iconic neon signs. The characteristic reddish-orange glow is produced by excited neon atoms releasing energy as photons.
-
Helium-Neon Lasers: Mixtures of helium and neon gas are commonly used in lasers. The precise energy levels within the neon atom, dictated by its 10 protons and electrons, enable the production of coherent, monochromatic laser light.
-
Cryogenics: Liquid neon, achievable due to its low boiling point, finds applications in cryogenics, the study and application of low temperatures. Its inertness makes it suitable for cooling sensitive equipment without unwanted chemical reactions.
Beyond the Protons: A Holistic View of Neon
While the number of protons is crucial for defining neon, a complete understanding requires considering other aspects of its atomic structure and behavior. This includes:
-
Electron Configuration: Neon's electron configuration (1s²2s²2p⁶) explains its chemical inertness. The filled outer shell means it lacks the tendency to gain or lose electrons to form chemical bonds.
-
Isotopic Abundance: The relative abundance of Neon-20, Neon-21, and Neon-22 influences the average atomic mass of neon, a value used in various calculations.
-
Spectroscopy: The study of the light emitted or absorbed by neon atoms allows scientists to further analyze its energy levels and confirm the arrangement of its electrons, which is directly related to its proton count.
Conclusion: The Importance of Atomic Structure
The seemingly simple question of "how many protons does neon have?" opens a door to a deeper understanding of atomic structure, chemical properties, and the remarkable applications of elements in our daily lives. The 10 protons in neon's nucleus are not just a number; they are the foundation upon which its unique properties and significant role in various technologies are built. This comprehensive exploration highlights the interconnectedness of atomic structure and the macroscopic world, emphasizing the importance of fundamental scientific principles in explaining the behavior of matter. The knowledge gained extends beyond simple atomic characteristics; it underscores the power of scientific inquiry to unlock the secrets of the universe, one element at a time.
Latest Posts
Latest Posts
-
75 Is What Percent Of 150
Mar 08, 2025
-
2 3 4 In Decimal Form
Mar 08, 2025
-
Factor X 3 3x 2 3
Mar 08, 2025
-
What Is 41 Celsius In Fahrenheit
Mar 08, 2025
-
Multiplos Comunes De 6 Y 9
Mar 08, 2025
Related Post
Thank you for visiting our website which covers about How Many Protons Does Neon Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
